Abstract
Introduction: In September 2013, the Spanish Myeloma Group (PETHEMA/GEM) activated a randomized phase III trial of induction therapy with VRD-GEM for 6 cycles (with extended full dose of lenalidomide from days 1 to 21), followed by ASCT conditioned with Mel-200 versus BuMel (intravenous buslfan 9.6 mg/kg plus MEl-140) and post-transplant consolidation with two cycles of VRD-GEM in patients 65 years or younger with newly diagnosed symptomatic MM.
Aim:Tthe objectives of the study were to evaluate CR rate after induction, post-transplant and post-consolidation, evaluation of minimal residual disease (MRD) after each treatment step, progression-free survival, overall survival and toxicity. We report here the response rate, including MRD assessment and toxicity after the induction therapy, ASCT and consolidation, as well as stem cell movilization data.
Patients and methods: VRD-GEM consisted of bortezomib 1.3 mg/m2/sc on days 1,4,8,11 of each cycle, lenalidomide 25 mg/day on days 1 to 21 and dexamethasone 40 mg on days 1-4 and 9-12 at 4-week intervals for 6 cycles. Mobilization was performed after the third induction cycle. From September 18, 2013 to November 16, 2015, 458 eligible patients were included (male 240; female: 218; median age 58 years) . The M-protein type was IgG 60%, IgA 23%, light-chain 15%, IgD 1% and non-secretory 1%. The ISS stage was I in 39% of the patients, II in 36% , III in 23% and non-available in 2%. Soft-tissue plasmacytomas (EMP) was reported in 102 (22%) patients. Twenty percent of the patients had high-risk cytogenetics (t (4;14), t(14;16) and/or 17p deletion). MRD was analyzed using next-generation flow (NGF) following EuroFlow standard operation protocols as defined by the IMWG; the sensitivity level for MRD detection was of 3x10-6. Investigator assessment of response was analyzed base on all eligible patients. Response will be further assessed by an external Independent Response Adjudication Commitee.
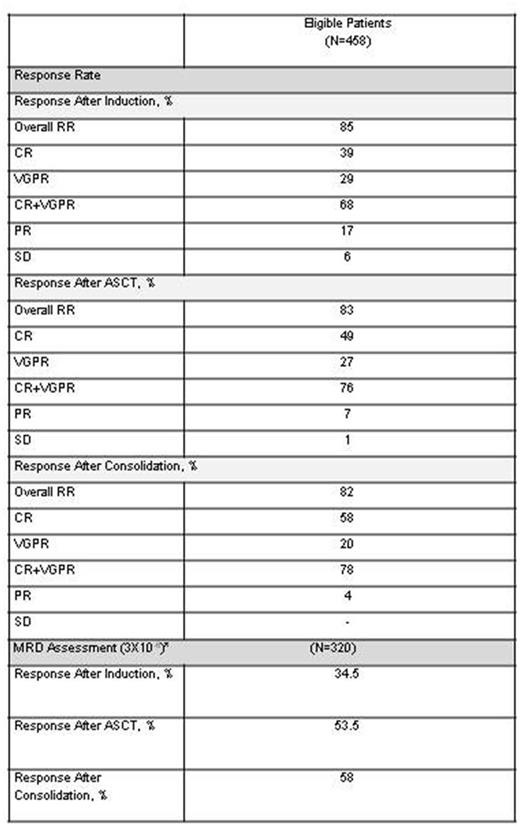
Results: The overall response rate at the end of induction therapy was 85%, including 39% CR (sCR 28%, 11% CR), 29% VGPR and 17% PR (Table 1). Stable disease (SD) and progressive disease (PD) during induction were 6% each. There were 12 patients (3%) with missing data for response evaluation. Patients with high-risk cytogenetics had a CR rate of 46% (stringent CR 34%, CR 12%) and 13% of PD. Patients with 17p deletion had a trend towards a higher rate of PD (20%) during induction when compared with other high-risk cytogentic abnormalities (t (4;14): 13%, t(14;16) 12%, 1p deletion 10%,). Grade 3-4 neutropenia and thrombocytopenia was reported in 11% and 6% of the patients, respectively. The most frequent grade 3-4 non-hematological toxicities were liver (4%) and skin (3%). The frequency of grade 3 peripheral neuropathy was only 1%, with 22% and 13% grade I and grade II, respectively. No patient developed grade 4 peripheral neuropathy. Dose reductions due to toxicity were needed in 31% of the patients (18% bortezomib reduction, 10% of lenalidomide and 3% of dexamethasone). Sixty-one (13%) patients were discontinued before ASCT due to the following reasons: PD: 32 patients (19 between the end of induction and transplant), toxicity: 9, death: 7 and other reasons: 13. The median collected CD34-positive cells was 4.66x106/Kg. 86% of the patients required only one stem cell mobilization while 13% required two or more. There were only two stem cell mobilization failures.
The ORR after ASCT was 83%, including 49% CR (36% sCR, 13% CR), 27% VGPR and 7% PR. After consolidation the CR rate increased up to 58% (46% sCR, 12% CR). Detailed analyses of MRD kinetics in 320 patients with available MRD measurements at the 3 planned time points (after induction,ASCT and consolidation), showed that the percentage of MRD-negative patients increased from 34.5% to 53.5% and 58%, respectively.
Conclusions: 1) VRD-GEM with full dose of lenalidomide from days 1 to 21 is a highly effective pretransplant induction régimen, 2) toxicity, particularly peripheral neuropathy, is remarkably low compared to our prior study with VTD (Rosiñol et al, Blood 2012), 3) stem cell mobilization after 3 cycles of VRD-GEM is highly effective, 4) responses continue to deepen throughout treatment with 58% of patients achieving MRD-negativity post-consolidation, and 5) the high response quality is confirmed by a MRD negative rate using NGF up to 58% after consolidation. Study data are not yet mature to evaluate the primary endpoint of PFS.
Rosinol: Janssen: Honoraria; Celgene: Honoraria. Oriol: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Celgene: Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia. Sureda: Janssen: Honoraria. Paiva: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; EngMab: Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Amgen: Honoraria. Mateos: Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. San Miguel: Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Lahuerta: Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Blade: Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal